Soap

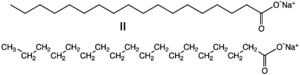
In chemistry, soap is a salt of a fatty acid.[1] Soap is mainly used for washing and cleaning, but soaps are also important components of lubricants.
Soaps for cleansing are obtained by the treating vegetable or animal oils and fats with a strongly alkaline solution. The alkaline solution, often lye, promotes what is known as saponification. In saponification, fats are broken down (hydrolyzed) yielding crude soap, i.e. impure salts of fatty acids and glycerol.
Contents |
Soaps for lubrication greases
Soaps are key components of most lubrication greases, which are usually emulsions of calcium and lithium soaps in a mineral oil. Lithium-based greases are particularly important. Many other metal ions are used, including aluminium, sodium, and mixtures of various metal ions. Such soaps are sometimes classified as thickeners, meaning that they elevate the viscosity of the oil. In ancient times, lubricating greases were prepared by the addition of lime to olive oil.[2]
Mechanism of cleansing soaps
When used for cleaning, soap serves as a surfactant in conjunction with water. The cleaning action of this mixture is attributed to the action of micelles, tiny spheres coated on the outside with polar carboxylate groups, encasing a hydrophobic (lipophilic) pocket that can surround the grease particles, allowing them to dissolve in water. The hydrophobic portion is made up of the long hydrocarbon chain from the fatty acid. In other words, whereas normally oil and water do not mix, the addition of soap allows oils to dissolve in water, allowing them to be rinsed away. Synthetic detergents operate by similar mechanisms to soap.

Effect of the alkali
The nature of the soap depends on the alkali metal. Sodium soaps, prepared from sodium hydroxide are firmer. Potassium soaps, derived from potassium hydroxide are softer or often liquid. Historically, potassium hydroxide was extracted from the ashes of bracken or other wood ashes. Lithium soaps also tend to be harder - these are used exclusively in greases.
Effects of fats

Soap are derivatives of fatty acids. Traditionally soaps are derived from triglycerides (vegetable and animal fats). Triglyceride is the technical name for these triesters of fatty acids. Sodium tallowate, a common ingredient in many soaps, is derived from rendered beef fat. Typical vegetable oils used in soap making are palm oil, where the product is typically softer. If soap is made from pure olive oil it may be called Castile soap or Marseille soap. The term "Castile" is also sometimes applied to soaps with a mix of oils, but a high percentage of olive oil.
Aside from olive oil, other saponifiable oils and fats include coconut, palm, cocoa butter, hemp oil, and shea butter to provide different qualities. For example, olive oil provides mildness in soap. Coconut oil provides lots of lather, whereas coconut and palm oils provide hardness. Sometimes castor oil can also be used as an ebullient. Most common, though, is a combination of coconut, palm, and olive oils. Smaller amounts of unsaponifiable oils and fats that do not yield soap are sometimes added for further benefits.
History of cleansing soaps
Early history
The earliest recorded evidence of the production of soap-like materials dates back to around 2800 BC in Ancient Babylon.[3] In the reign of Nabonidus (556-539 BCE) a recipe for soap consisted of uḥulu [ashes], cypress [oil] and sesame [seed oil] "for washing the stones for the servant girls".[4] A formula for soap consisting of water, alkali and cassia oil was written on a Babylonian clay tablet around 2200 BC.
The Ebers papyrus (Egypt, 1550 BC) indicates that ancient Egyptians bathed regularly and combined animal and vegetable oils with alkaline salts to create a soap-like substance. Egyptian documents mention that a soap-like substance was used in the preparation of wool for weaving.
Roman history
The word sapo, Latin for soap, first appears in Pliny the Elder's Historia Naturalis, which discusses the manufacture of soap from tallow and ashes, but the only use he mentions for it is as a pomade for hair; he mentions rather disapprovingly that among the Gauls and Germans men are likelier to use it than women.[5]
A popular belief encountered in some places claims that soap takes its name from a supposed "Mount Sapo" (q.v.); but there is no such place, and no evidence for the apocryphal story. In fact, the Latin word sapo simply means "soap"; it was likely borrowed from an early Germanic language, and is cognate with Latin sebum, "tallow", which appears in Pliny the Elder's account.[6] Roman animal sacrifices usually burned only the bones and inedible entrails of the sacrificed animals; edible meat and fat from the sacrifices were taken by the humans rather than the gods. Animal sacrifices in the ancient world would not have included enough fat to make much soap.
Zosimos of Panopolis c. 300 AD describes soap and soapmaking.[7] Galen describes soap-making using lye and prescribes washing to carry away impurities from the body and clothes. According to Galen, the best were German and ones from Gaul were second best. This is a reference to true soap in antiquity.[7]
Medieval history
Soap-makers in Naples were members of a guild in the late sixth century,[8] and in the 8th century, soap-making was well-known in Italy and Spain.[9] The Carolingian capitulary De Villis, dating to around 800, and sometimes attributed to Charlemagne, mentions soap as being one of the products the stewards of estates are to tally. Soap-making is mentioned both as "women's work" and the produce of "good workmen" alongside other necessities such as the produce of carpenters, blacksmiths and bakers.[10]
Soaps made from vegetable oils (such as olive oil), aromatic oils (such as thyme oil) and lye (al-Soda al-Kawia) were first produced by chemists. From the beginning of the 7th century, soap was produced in Nablus (West Bank), Kufa (Iraq) and Basra (Iraq). Soap was perfumed and colored, some of the soaps were liquid and others were solid. They also had special soap for shaving. It was sold for 3 Dirhams (0.3 Dinars) a piece in 981 AD. The Persian chemist Al-Razi wrote a manuscript on recipes for true soap. A recently discovered manuscript from the 13th century details more recipes for soap making; e.g. take some sesame oil, a sprinkle of potash, alkali and some lime, mix them all together and boil. When cooked, they are poured into molds and left to set, leaving hard soap.
15th-20th Centuries

In France, by the second half of the 15th century the semi-industrialized professional manufacture of soap was concentrated in a few centers of Provence— Toulon, Hyères and Marseille— which supplied the rest of France.[11] In Marseilles, by 1525, production was concentrated in at least two factories, and soap production at Marseille tended to eclipse the other Provençal centers.[12] English manufacture tended to concentrate in London.[13]
Finer soaps were later produced in Europe from the 16th century, using vegetable oils (such as olive oil) as opposed to animal fats. Many of these soaps are still produced, both industrially and by small scale artisans. Castile soap is a popular example of the vegetable-only soaps derived by the oldest "white soap" of Italy.
In modern times, the use of soap has become universal in industrialized nations due to a better understanding of the role of hygiene in reducing the population size of pathogenic microorganisms. Industrially manufactured bar soaps first became available in the late eighteenth century, as advertising campaigns in Europe and the United States promoted popular awareness of the relationship between cleanliness and health.[14]

Until the Industrial Revolution, soapmaking was conducted on a small scale and the product was rough. Andrew Pears started making a high-quality, transparent soap in 1789 in London. His son-in-law, Thomas J. Barratt, opened a factory in Isleworth in 1862. William Gossage produced low-price good quality soap from the 1850s. Robert Spear Hudson began manufacturing a soap powder in 1837, initially by grinding the soap with a mortar and pestle. American manufacturer Benjamin T. Babbitt introduced marketing innovations that included sale of bar soap and distribution of product samples. William Hesketh Lever and his brother, James, bought a small soap works in Warrington in 1885 and founded what is still one of the largest soap businesses, now called Unilever. These soap businesses were among the first to employ large scale advertising campaigns.
Traditional soap making
The most popular soapmaking process today is the cold process method, where fats such as olive oil react with lye, while some soapers use the historical hot process.
Handmade soap differs from industrial soap in that, usually, an excess of fat is used to consume the alkali (superfatting), and in that the glycerin is not removed, leaving a naturally moisturizing soap and not pure soap. Superfatted soap, soap which contains excess fat, is more skin-friendly than industrial soap, though if too much fat is added, it can leave users with a "greasy" feel to their skin. Sometimes an emollient such as jojoba oil or shea butter is added "at trace" (the point at which the saponification process is sufficiently advanced that the soap has begun to thicken) in the belief that it will escape the saponification and remain intact, or in the case of hot process soap - after most of the oils have saponified so that they remain unreacted in the finished soap. Superfatting can also be accomplished through a process called a lye discount, where, instead of putting in extra fats, the soap maker puts in less lye.
Process
In both cold-process and hot-process soapmaking, heat may be required for saponification.
Cold-process soapmaking takes place at a sufficient temperature to ensure the liquefication of the fat being used. The lye and fat may be kept warm after mixing to ensure that the soap is completely saponified.
Unlike cold-processed soap, hot-processed soap can be used right away because lye and fat saponify more quickly at the higher temperatures used in hot-process soapmaking.
Hot-process soapmaking was used when the purity of lye was unreliable, and this process can use natural lye solutions, such as potash. The main benefit of hot processing is that the exact concentration of the lye solution does not need to be known to perform the process with adequate success.
Cold-process soapmaking requires exact measurements of lye and fat amounts and computing their ratio, using saponification charts to ensure that the finished product is mild and skin-friendly. Saponification charts can also be used in hot-process soapmaking, but are not as necessary as in cold-process soapmaking.
- Hot process
In the hot-process method, lye and fat are boiled together at 80–100 °C until saponification occurs, which before modern thermometers, the soapmaker determined by taste (the bright, distinctive taste of lye disappears once all the lye is saponified) or by eye; the experienced eye can tell when gel stage and full saponification have occurred. Beginners can find this information through research, and classes. It is highly recommended to not "taste" your soap for lye for readiness. Lye, when not saponified is a highly caustic material. Rather, research proper Hot Process techniques and use a digital or analog sugar thermometer to make sure you are at the right temperature.
After saponification has occurred, the soap is sometimes precipitated from the solution by adding salt, and the excess liquid drained off. The hot, soft soap is then spooned into a mold.
- Cold process
A cold-process soapmaker first looks up the saponification value of the fats being used on a saponification chart, which is then used to calculate the appropriate amount of lye. Excess unreacted lye in the soap will result in a very high pH and can burn or irritate skin. Not enough lye, and the soap is greasy. Most soap makers formulate their recipes with a 4-10% deficit of lye so that all of the lye is reacted and that excess fat is left for skin conditioning benefits.
The lye is dissolved in water. Then oils are heated, or melted if they are solid at room temperature. Once both substances have cooled to approximately 100-110 °F (37-43 °C), and are no more than 10°F (~5.5°C) apart, they may be combined. This lye-fat mixture is stirred until "trace" (modern-day amateur soapmakers often use a stick blender to speed this process). There are varying levels of trace. Depending on how additives will affect trace, they may be added at light trace, medium trace or heavy trace. After much stirring, the mixture turns to the consistency of a thin pudding. "Trace" corresponds roughly to viscosity. Essential oils, fragrance oils, botanicals, herbs, oatmeal or other additives are added at light trace, just as the mixture starts to thicken.
The batch is then poured into molds, kept warm with towels, or blankets, and left to continue saponification for 18 to 48 hours. Milk soaps are the exception. They do not require insulation. Insulation may cause the milk to burn. During this time, it is normal for the soap to go through a "gel phase" where the opaque soap will turn somewhat transparent for several hours, before once again turning opaque. The soap will continue to give off heat for many hours after trace.
After the insulation period the soap is firm enough to be removed from the mold and cut into bars. At this time, it is safe to use the soap since saponification is complete. However, cold-process soaps are typically cured and hardened on a drying rack for 2–6 weeks (depending on initial water content) before use. If using caustic soda it is recommended that the soap is left to cure for at least four weeks.
- Molds
Many commercially available soap molds are made of silicone or various types of plastic, although many soap making hobbyists may use cardboard boxes that are lined with plastic wrap. Soaps can be made in long loaves that are cut into individual bars, block molds that are cut into loaves and then bars, or individual molds.
Purification and finishing
The common process of purifying soap involves removal of sodium hydroxide, glycerol and some impurities. These components are removed by boiling the crude soap curds in water and re-precipitating the soap with salt.
Most of the water is then removed from the soap. This was traditionally done on a chill roll which produced the soap flakes commonly used in the 1940s and 1950s. This process was superseded by spray dryers and then by vacuum dryers.
The dry soap (approximately 6-12% moisture) is then compacted into small pellets. These pellets are now ready for soap finishing, the process of converting raw soap pellets into a saleable product, usually bars.

Soap pellets are combined with fragrances and other materials and blended to homogeneity in an amalgamator (mixer). The mass is then discharged from the mixer into a refiner which, by means of an auger, forces the soap through a fine wire screen. From the refiner the soap passes over a roller mill (French milling or hard milling) in a manner similar to calendering paper or plastic or to making chocolate liquor. The soap is then passed through one or more additional refiners to further plasticize the soap mass. Immediately before extrusion it passes through a vacuum chamber to remove any trapped air. It is then extruded into a long log or blank, cut to convenient lengths, passed through a metal detector and then stamped into shape in refrigerated tools. The pressed bars are packaged in many ways.
Sand or pumice may be added to produce a scouring soap. The scouring agents serve to remove dead skin cells from the surface being cleaned. This process is called exfoliation. Many newer materials are used for exfoliating soaps which are effective but do not have the sharp edges and pore size distribution of pumice.
Nanoscopic metals are commonly added to certain soaps specifically for both coloration and anti-bacterial properties. Titanium powder is commonly used in extreme "white" soaps for these purposes; nickel, aluminium and silver are less commonly used. These metals provide electron-robbing behavior when in contact with bacteria, stripping electrons from the organism's surface and thereby disrupting their functioning (typically killing the bacteria when it has lost too many electrons). Because some of the metal is left behind on the skin and in the pores, the benefit can also extend beyond the actual time of washing, helping reduce bacterial contamination and reducing potential odors from bacteria on the skin surface.
See also
- Foam
- Soap bubble
- Soap dish
- Soap dispenser
- Soap substitute
References
- ↑ IUPAC. "IUPAC Gold Book - soap" Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8. doi:10.1351/goldbook. Accessed 2010-08-09
- ↑ Thorsten Bartels et al. "Lubricants and Lubrication" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Weinheim. doi:10.1002/14356007.a15 423
- ↑ Willcox, Michael (2000). "Soap". In Hilda Butler. Poucher's Perfumes, Cosmetics and Soaps (10th edition ed.). Dordrecht: Kluwer Academic Publishers. pp. 453. "The earliest recorded evidence of the production of soap-like materials dates back to around 2800 BCE in ancient Babylon."
- ↑ Noted in Martin Levey, "Gypsum, salt and soda in ancient Mesopotamian chemical technology" Isis 49.3 (September 1958:336-342) p. 341.
- ↑ Pliny the Elder, Natural History, XXVIII.191.
- ↑ http://www.etymonline.com/index.php?term=soap
- ↑ 7.0 7.1 Partington, James Riddick; Bert S Hall (1999). A History of Greek Fire and Gun Powder. JHU Press. pp. 307. ISBN 0801859549.
- ↑ footnote 48, p. 104, Understanding the Middle Ages: the transformation of ideas and attitudes in the Medieval world, Harald Kleinschmidt, illustrated, revised, reprint edition, Boydell & Brewer, 2000, ISBN 0-85115-770-X.
- ↑ p. 632, chapter 11, Anionic and Related Lime Soap Dispersants, Raymond G. Bistline, Jr., in Anionic surfactants: organic chemistry, Helmut Stache, ed., Volume 56 of Surfactant science series, CRC Press, 1996, ISBN 0-8247-9394-3.
- ↑ Robinson, James Harvey (1904). Readings in European History: Vol. I. Ginn and co. http://www.fordham.edu/halsall/source/carol-devillis.html.
- ↑ John U. Nef, "A Comparison of Industrial Growth in France and England from 1540 to 1640: III" The Journal of Political Economy 44.5 (October 1936:643-666) p. 660ff.
- ↑ L. Barthélemy, "La savonnerie marseillaise", 1883, noted by Nef 1936:660 note 99.
- ↑ Nef 1936:653, 660.
- ↑ McNeil, Ian (1990). An Encyclopaedia of the history of technology. Taylor & Francis. pp. 2003–205. ISBN 9780415013062. http://books.google.de/books?id=uxsOAAAAQAAJ&pg=PA203.
Further reading
- Garzena, Patrizia - Tadiello, Marina (2004). Soap Naturally - Ingredients, methods and recipes for natural handmade soap. Programmer Publishing. ISBN 978-0-9756764-0-0
External links
- Soap History The Soap and Detergent Association
- A short history of soap The Pharmaceutical Journal
- Medieval Sourcebook: The Capitulary De Villis
